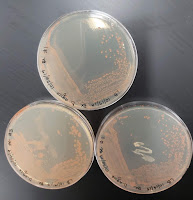
10/1 Today, Chad and I went through our box of DNA isolations and PCR products. We've been working on isolating and running PCR on each of the Deinococcus species we have so that we can have 16s rRNA sequencing done on them. We decided that since we've had these for some time now, that Chad would run new PCR for the isolations that we already have. Then we'll only need the last two species that we currently have to be grown up, DNA isolated, and PCR run. 10/3 Today I had to make more R2B broth media, and once it was autoclaved, I inoculated from plate to flasks and placed them in the incubator. 10/4 Today, I did some planning and prep for Saturday's inoculations. John and I also mapped out an idea for a growth media database. 10/5 Today, the flasks were removed from the incubator and inspected for growth. All six showed growth, and four of the six showed biofilm formation on the sides of the flasks. Then, John an